close this article
You can also choose to view the scanned page images that comprise this article.
This article is copyright Derek W. Sears, it originally appeared in the Journal of the
British Astronomical Association, Volume 86, pages 133-9, and is reproduced here by
permission of the author.
Edward Charles Howard and an early British contribution to meteoritics
by Derek W. Sears
The Honourable Edward Charles Howard discovered that stony meteorites, as
well as irons, contained nickel. This major finding was acknowledged by
Gustav Rose in 1863 when he introduced the term Howardites for one of the
classes of meteorites. Howard concluded from this finding that meteorites
could not come from the Earth. It had been suggested that certain stones and
irons were associated with meteors, but the scientific world was very sceptical.
It is the purpose of this paper to review Howard's work on meteorites and to
present the biographical details I have been able to locate.
The Complete Peerage states that Howard's father, Henry Howard of Glossop,
County Derby, was an unsuccessful wine merchant in Dublin.[1] After his failure
in business his creditors were paid by the ninth Duke of Norfolk, a distant
relative, who subsequently appointed Howard's father agent at his Sheffield
estates. Henry Howard and his wife Juliana moved to Darnall Hall, Sheffield,
and on 1774 May 28 Edward Charles was born. He was the youngest of three
sons, the others being Bernard Edward (who became the twelfth Duke of
Norfolk) and Henry Thomas. After seven years at Darnall Hall the family
moved to Heath Hall, Wakefield.
In 1783 Edward joined his brothers at the English College of Douai in
France.[2] At that time it was thought impossible to receive a good Catholic
education in Protestant England; consequently, numerous schools were established
on the Continent and staunch Roman Catholic families, such as the
Howard's, sent their sons there. Howard was rather young when he started in
the "Refiqui" school at Douai, being nine instead of the more usual 13. On
1788 March 10, by which time he had completed less than half the course, he
returned to England. His father had died six months previously and this may
have been the reason for his leaving: alternatively, early signs of the French
revolution, which was to break out a year later, may have prompted his
departure.
Over the next few years Howard became a skilful chemist. In 1799 he was
elected a Fellow of the Royal Society and also became a member of the Royal
Society of Arts. Election to the Royal Society then required that a certificate,
signed by six Fellows of the Society, be on display for six months; the certificate
stated that the applicant was suitable and would become a "useful and valuable
member" of the Society. Howard's application was balloted and he was elected
on 1799 January 17. Among the signatories to his certificate were the eleventh
Duke of Norfolk, Richard Pearson, George Shaw, John Abernethy and Charles
Hatchett. Both Pearson and Shaw were very interested in meteorites, as is
evident from their lengthy footnote to the Philosophical Transactions Abridgements
published in 1809.[3] Abernethy was involved in Howard's work on
mercury fulminate in 1800, and Hatchett with his meteorite studies in 1802.
Hatchett, an eminent chemist and the discoverer of niobium, was probably a
personal friend of Howard's, and he not only signed the certificate but also
chaired a committee of which Howard was a member. This committee was the
Chemical Committee of the Royal Institution, and was authorized to ". . . make
such experiments in the laboratory of this Institution as they think fit".[4] Perhaps
this may have included experiments on stones "said to have fallen to the Earth".
In 1804 they were also to become involved together in the disputed discovery
of palladium.[5]
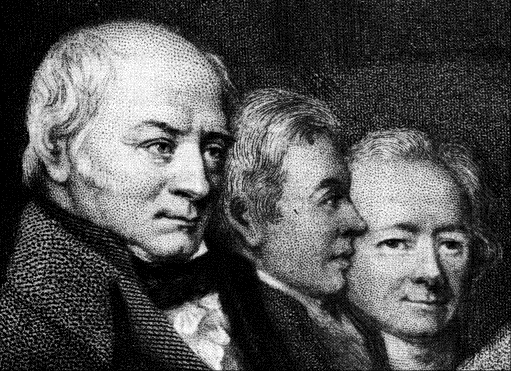
FIGURE 1. Edward Howard (centre) as he appears in an engraving at the
Royal Institution. On either side of Howard are two of his contemporaries,
the geologist William Smith (left) and the chemist William Allen. The
engraving is a copy of a profile in bronze probably made posthumously.
(Royal Institution Photograph.)
During the six months prior to his election, Howard was working on the
nature of muriatic acid (hydrochloric acid). This resulted in his discovery of
mercury fulminate, an extremely unstable substance which is too violent for use
as the explosive in munitions.[6] Howard suffered many injuries from these
experiments, but his work was acknowledged by the award of the Copley Medal
for the year 1800. In his address at the presentation of the Medal, Sir Joseph
Banks, the President of the Royal Society, summarized Howard's work and
also introduced the next topic of his studies:
"Mr. Howard has not stopped here. He has announced to us the discovery of a
fulminating silver, analogous in some degree to his fulminating mercury, and he is
now employed in the analysis of certain stones, generations in the air by fiery
meteors, the component parts of which will probably open a new field of speculation
and discussion to mineralogists as well as to meteorologists".[7]

FIGURE 2. Part of the first page of Howard's manuscript concerning
meteorites. The title and the first line had been altered to moderate the
tone. For example, "substances which have at different times fallen" has
been altered to ". . . are said to have fallen".
This passage not only reveals Howard's activies at this time but also Bank's
own attitude towards "atmospheric stones",[8] and the role of the President in
Howard's meteorite studies was probably an extremely important one. Near
the beginning of his meteorite paper, Howard remarked that it was the President
who first observed the similarity between two recently-fallen meteoritic
stones.
The similarity between stones which had fallen in various countries was the
basic theme of Howard's meteorite paper.[9] For example, near the beginning
Howard discussed the report of a commission of the Paris Academy on the Luce
meteorite. This commission, which included Lavoisier, reported that the stone
was no more than terrestrial pyrite struck by lightning. Howard was very
scornful, remarking that the academicians must think ". . . that the lightning
had fallen by preference on pyritical matter", since they must have known of
at least two similar stones.
His paper was divided into two main sections, the first being concerned with
stony meteorites and the second with irons. The stony meteorites Howard had
acquired were from Wold Cottage, Yorkshire; Siena, Italy; Benares, India; and
Tabor, Bohemia. The first two were given to him by Banks. Benares was sent
to Howard by John Lloyd Williams, a Fellow of the Royal Society who lived
in India. Williams sent some lengthy notes to Banks describing the fall, and
Howard included these in the paper. Tabor was loaned by Charles Greville, a
well-known mineralogist whose collection reached such proportions that after
his death in 1809 the British Museum had to find £14 000 for its purchase.[14]
The value of collecting these "stones from the sky" was very uncertain at that
time. This was the age of enlightenment--when medieval superstitions were
being laid low. For example, Howard was very critical of the "fairground"
manner in which fragments of the Wold Cottage meteorite and testimonials to
its fall were exhibited. The exhibition was situated ". . . in the Gloucester coffee
house, Picadilly" and entry could be purchased for one shilling.[15] Tabor's
previous owner once threw out his entire collection of meteorites because he
feared he might suffer scorn for preserving it.[16]
Before presenting his own chemical experiments Howard presented descriptions
of the stones by Jacques Louis Compte de Bournon.[17] Bournon (1751-1825) was
one of the foremost mineralogists of the time. He had been lecturing
in England since 1792, and he had been consulted on mineralogical matters by
Banks. Bournon distinguished four kinds of material in the meteorites: "martial
pyrites", iron, earthy cement and curious globules. The existence of metal and
pyrite (an iron sulphide) had been previously noted by Dominic Troili in the
Albereto Stone in 1766. The globules, now known as chondrules, were an
entirely new discovery. Chondrules are unique to meteorites and therefore very
significant to theories concerning their origin. Howard was obviously worried
about the existence of these many kinds of material. He remarked about the
Luce analysis:
"It was unfortunately made on an aggregate portion of the stone, and not of each
distinct substance, irregularly disseminated through it. The proportions obtained
were, consequently, as accidental as the arrangement of every substance in the
mass."[10]
It is for this reason that meteorites are still considered notoriously difficult
to analyze. The main factor in Howard's success in discovering nickel is that
he chose to examine each material separately. In the iron grains he discovered
up to 25% nickel. He observed the "pyrite" to be distinct from terrestrial pyrite,
but it was 1863 before Van Haidinger named the mineral troilite. Howard
wrote:
"Upon the whole, however, it may be concluded, that these pyrites are of a very
particular nature; for, although Henkel had observed that sulphur may be separated
from pyrites by muriatic acid, it is by no means the normal habitude of pyrites to
be of such an easy decomposition."[11]
He then gave an analysis of the globular bodies and of the earthy cement.
Finally Howard compared his findings with previous analyses; Barthold's of
the Ensisheim stone and the academicians of Luce. Barthold's analysis had
yielded silica, alumina, iron oxide and lime. The academicians had only reported
sulphur, iron and vitrifiable matter (silica). Howard had not found lime and
alumina, but he did discover nickel.[18]
Nickel is an extremely important component, since it is very seldom present
in terrestrial rocks in anything but trace amounts. Its comparative abundance
in meteorites therefore distinguishes them from terrestrial rocks and proves they
cannot have come from the Earth. This argument was made all the more
convincing by the fact that the French chemist Proust had discovered nickel in
"native irons" (now known to be iron meteorites), and the legends about them
collected by the German physicist Chladni frequently associated them with
meteors.
Howard concluded his section on stones with a brief discussion of possible
mechanisms for producing the light associated with meteors. This was a major
problem at the time and many theories involved the new electrical phenomena.
In this connection Howard recorded the first observation of the luminescence
of meteorites:
"I ought not to suppress, that in endeavouring to form an artificial black coating
on the interior surface of one of the stones from Benares, by sending over it the
electrical charge of about 37 square feet of glass it was observed to become luminous,
in the dark, for nearly a quarter of an hour; and that the tract of the electrical fluid
was rendered black."[12]
The black coating to which Howard refers is, of course, the fusion crust
produced by atmospheric heating.
The section on iron meteorites follows the same pattern as that on the stones.
The irons were obtained from Greville (the "Bohemian iron" and two pieces of
the "Pallas iron"--Krasnojarsk), from the British Museum (the "Otumpa
iron"--Campo del Cielo) and from Charles Hatchett (Siratik). First came a
description from Bournon. He observed that the Pallas iron was cellular, and
that in places the cells were filled with a yellow-green glass resembling peridot
(olivine, a silicate mineral which is an important constituent of most stony
meteorites). Stony-iron meteorites of this kind are now known as pallasites.
Howard then gave his analyses, confirming Proust's discovery that the irons
contained nickel.
Howard ended his paper with the comments:
"From these facts, I shall draw no conclusion, but submit the following
enquiries.
lst. Have not all fallen stones, and what are called native irons, the same origin?
2dly. Are all, or any, the produce or the bodies of meteors?"[13]
His published contribution was therefore the discovery of nickel in stones and
the unique character of meteoritic "pyrites". To these one may add Bournon's
finding of chondrules in the stones and the peridot-like glass in the pallasite.
There is, however, a further finding which appeared in the manuscript (now
in the Royal Society Archives) but did not reach the published article. In this
manuscript the following paragraph has been crossed out:
"To discover the cause of the deliquescence of several spots on the large specimen
of Mr. Greville he readily consented to its being immersed in distilled water, which
in the course of a few hours was very strongly impregnated with iron (, and which)
on examination with nitrate of silver a copious precipitate, insoluble in nitric acid,
was afforded. Hence the solution was a muriate of iron, and it may be presumed,
had formed by some accidental means."
This is clearly the alteration product of the mineral lawrencite (iron chloride),
a highly hygroscopic mineral discovered in 1855 by J. Lawrence Smith. The
same work as that described by Howard was performed independently nearly
40 years later by Jackson, who again did not realise that the source of the
droplets was a new mineral.[19] Inclusions of lawrencite constitute a major
problem for the curators of meteorites since the decomposition products attack
the rest of the iron. The Cranbourne meteorite, for example, has to be kept in
an atmosphere of dry nitrogen to prevent rapid destruction by this process The
paragraph in Howard's manuscript was probably removed by the author himself.
Additional phrases, crossed out in the original manuscript, make it clear that he
thought the droplets of iron chloride were produced by an accidental spillage
of hydrochloric acid.
The number of alterations in the original manuscript is very high. Frequently
they temper the style, so that assertions become suggested possibilities. Most
are probably Howard's, although it is difficult to be certain because they are
made with a broader nib. A few are almost certainly by Edward Whittaker
Gray, Secretary of the Royal Society, who crossed out in a characteristic way
by drawing a series of loops through the writing, thus making it difficult to read.
After the publication of the 1802 paper, Howard wrote nothing on the subject
of meteorites. Banks continued his interest, writing to Blagden in 1814 about
the Limerick meteorite (see ref. 8). It would seem, therefore, that if the
motivation for Howard's meteorite studies came from the President, it was sufficient
to sustain only one piece of work.
Within the next few years Howard married Elizabeth Maycock and became
the father of two daughters, Elizabeth and Julia, and a son, Edward Giles.
According to one account Howard's wife was the daughter of a London sugar
refiner, and it was this which led to his subsequent interest in the sugar industry.[20]
Another story is that Howard was asked to find a way of converting sugar into
manure as a means of relieving the overburdened warehouses and at the same
time avoiding sugar duty.[21] In any case, Howard became actively involved in
this industry, making several inventions and pioneering many new techniques.[23]
Probably his major invention was the vacuum pan which enabled distillation to
be conducted under reduced pressure and thus prevented discoloration of the
sugar by charring. It was patented in 1812 and was an immediate success,
remaining unchanged for many years. Howard was offered, and apparently
refused, £40 000 for the patent.[22] At the same time he also introduced
"Howard's Finings", a mixture of calcium oxide, alumina and calcium sulphate,
which was added to the syrup to precipitate organic impurities.
However, it was Howard's interest in the sugar industry which ultimately
caused his death. In 1816 he made a fatal visit to the oven room of his refinery.
This caused him to suffer a heat stroke, the effects of which led to his death
at Nottingham Place in London on 1816 September 28. He was buried at
St Pancras, Middlesex. His wife had died five years previously.
As we have seen, Howard's name features in the histories of the explosives
industry, the sugar industry and astronomy. He studied meteorites at a time
when it was highly uncertain as to how a piece of work on them would be
received, and no doubt Banks' support was much encouragement. Howard's
achievements were those of a very skilful chemist, and constitute a considerable
contribution to technology and to our knowledge of meteorites.
References
[1]
Doubleday, H. A., and Lord Howard de Walden, The Complete Peerage, 9, London, 1936.
[2]
Harris, P. R., Douai College Documents (Catholic Record Society, volume 63), London,
1972. I am grateful to Mr J. Bebbington of Sheffield City Libraries for information on
Howard's childhood.
[3]
Hutton, C., et al., Phil. Trans. Abridgements, 6 (1713-1723), 99, London, 1809.
[4]
Greenaway, F. (Ed.), The Archives of the Royal Institution of Great Britain in facsimile
Minutes of the Managers' Meetings 1799-1900, 2, 196, London, 1971.
[5]
White, A. M., and Friedman, H. B., J. Chem. Educ., 9, 236 (1932).
[6]
Howard, E. C., Phil. Trans., 90, 204 (1802). The historical background and additional
references to Howard's fulminating mercury work are given in Partington, J. R., A History
of Chemistry, 4, 257, London, l964.
[7]
Copy Minute Book of the Royal Society, 37 (1800-1802), 1800 December 1. I am grateful
to the Librarian of the Royal Society for access to this and to the other Royal Society
documents mentioned.
[8]
This phrase was used by Banks in a letter to Blagden, 1814 September 8. In the Royal
Society Archives (PSb50).
[9]
Howard, E. C., Phil. Trans., 92, 168 ( 1802). The manuscript is in the Royal Society Archives
(LP XII 16, 17, 18 and 19, volume 111).
[10]
Ibid., 187.
[11]
Ibid., 191.
[12]
Ibid., 201.
[13]
Ibid., 2l2.
[14]
Hey, M., Catalogue of Meteorites, lix, London, 1966.
[15]
Topham, E., Gentleman's Magazine, 67, 549 (1797).
[16]
Krinov, E. L., Principles of Meteoritics, 10, Oxford, l960.
[17]
Gillespie, C. C. (Ed.), Dictionary of Scientific Biography, 2, 355, New York, 1970.
[18]
A modern analysis of stony meteorites for these substances is alumina 3%, magnesia 23%,
silica 34%, iron oxide 15%, lime 2%, iron 12%, sulphur 2% (36% in troilite) and nickel
2% (may be greater than 50% in the nickel-iron grains).
[19]
Jackson, C. T., Amer. J. Sci., 34, 333 (1838).
[20]
Deere in reference 23.
[21]
Walker, W., Memoirs of Distinguished Men of Science of Great Britain, Living 1807-8, 96,
London, 1862.
[22]
Banks to Blagden, 1813 January 26. In the Royal Society Archives (RSb47).
[23]
References to Howard's work in the sugar industry are: Scoffern, I., Manufacture of Sugar,
114, London, 1849; Fairrie, G., Sugar, 155-156, Liverpool, 1925; Deere, N., The History
of Sugar, 2, 559, 560, London, 1950; Lippmann, E. D. von, Geschichte des Zuckers, 367-370,
Leipzig, 1890; Lippmann, E. D. von, Geschichte des Zuckers, 2 Auflage, 631, 634-636,
Berlin, 1929. I am grateful to the Librarian of Tate and Lyle Ltd for helping me to locate
these references.